October 22, 20257 min
Retatrutide Phase 2 Results (2025): Weight Loss Data, Safety, and Next Steps
Clear summary of Retatrutide Phase 2 results: percent weight loss at 24 and 48 weeks, side effects, dose escalation, and what Phase 3 will test.
Retatrutide Phase 2 Results Explained: Key Findings and What Comes Next
Retatrutide is an investigational medicine for weight management. It acts on three hormone pathways at once: GLP‑1, GIP, and glucagon. In a Phase 2 clinical trial, people taking retatrutide lost a large amount of weight on average. This article explains what the study showed, how the drug was taken, common side effects, and what Phase 3 will try to confirm.
We use simple language so anyone can follow along. We also include a glossary and FAQs at the end.
Fast facts
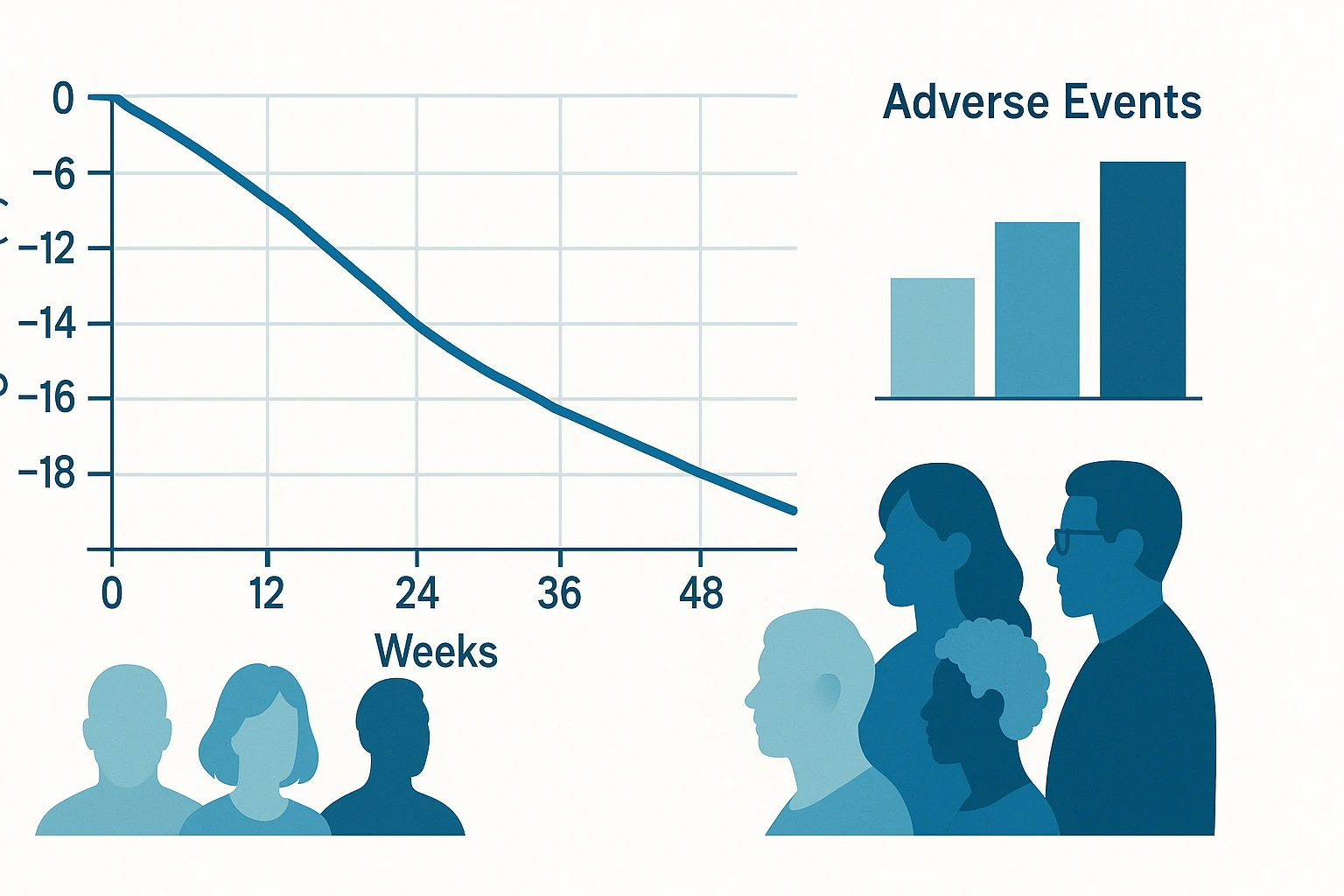
- Average weight loss: About 17.5% at 24 weeks and about 24.2% at 48 weeks with the 12 mg dose.
- Side effects: Mostly stomach‑related (nausea, vomiting, diarrhea, constipation), especially when the dose was being raised.
- Status: Retatrutide is not FDA‑approved. It is available only in clinical trials. Larger Phase 3 studies are running now to confirm results and evaluate long‑term safety.
Why retatrutide is different
Most weight‑loss medicines target one hormone pathway. Retatrutide targets three: GLP‑1, GIP, and glucagon. This three‑pronged approach aims to reduce appetite, improve blood sugar responses after meals, and potentially increase how much energy the body uses.
In simple terms, retatrutide helps you feel full, eat fewer calories, and may help your body burn a bit more energy.
The Phase 2 study design (what researchers did)
- Type of study: Randomized, double‑blind, placebo‑controlled (the gold standard for testing medicines).
- Who was included: Adults with obesity (BMI ≥30) or overweight (BMI ≥27) and at least one related health condition. People with diabetes were not included in this study.
- How long it lasted: 48 weeks.
- Doses tested: 1 mg, 4 mg, 8 mg, and 12 mg given once a week under the skin.
- Titration: Doses were increased slowly over 4–16 weeks to help people tolerate treatment.
- Main goal: Percent change in body weight at 24 weeks. Other goals extended to 48 weeks and included blood sugar and heart‑related measures.
Efficacy results (what people experienced)
The table below shows the average weight change at 24 and 48 weeks:
| Dose | Week 24 mean weight loss | Week 48 mean weight loss |
|---|---|---|
| 1 mg | ~7.2% | — |
| 4 mg | ~12.9% | — |
| 8 mg | ~17.3% | — |
| 12 mg | ~17.5% | ~24.2% |
What this means in everyday terms: if someone weighed 240 pounds at the start, 24.2% weight loss would be about 58 pounds by week 48 on the 12 mg dose.
Improvements beyond the scale
People taking retatrutide also saw improvements in several health measures:
- Blood sugar: Lower HbA1c and better fasting glucose in many participants.
- Blood pressure: More people reached normal blood pressure ranges.
- Liver health: Reductions in liver fat in some participants.
These changes are important because obesity increases the risk of type 2 diabetes, heart disease, and fatty liver disease.
Side effects and safety profile
Most side effects were gastrointestinal and tended to be worse during the first weeks of each dose increase. Many people felt better after staying at the same dose for a few weeks.
- Common side effects: nausea, vomiting, diarrhea, constipation, abdominal discomfort.
- Heart rate: Increases of around 5–10 beats per minute were seen at higher doses. The clinical importance of this needs further study.
- Discontinuations: Some people stopped the medicine because of side effects, more often at higher doses. This pattern is similar to other medicines in the same class.
Call your clinic if you have severe stomach pain, signs of pancreatitis, or symptoms that worry you.
Why dose escalation matters
Raising the dose slowly is important to help the body adapt. In the study, the dose was increased in steps over several weeks. Many doctors use similar slow titration outside of clinical trials with other medicines in this class to reduce nausea and vomiting.
Tips that may help during dose increases
- Eat small, balanced meals and avoid greasy foods.
- Stay hydrated. If you have vomiting or diarrhea, consider oral rehydration solutions.
- Pause dose increases if side effects are strong and call your clinician.
- Focus on protein and fiber to support fullness and muscle maintenance.
How retatrutide compares to other options (context)
Retatrutide’s average weight loss at 48 weeks in Phase 2 (about 24%) is higher than many other obesity medicines. For context:
- Wegovy (semaglutide): About 15% average weight loss at 68 weeks in the STEP 1 trial.
- Zepbound (tirzepatide): About 15–21% average weight loss at 72 weeks in the SURMOUNT‑1 trial.
These are different studies with different designs and participants. There were no direct head‑to‑head comparisons in the Phase 2 retatrutide trial, so we should be cautious when comparing across trials.
What Phase 3 will try to answer
- Can the weight loss be repeated and sustained in larger groups and over longer periods (72+ weeks)?
- What is the best dose and titration speed to balance benefits and side effects?
- What are the long‑term safety signals, including rare side effects and cardiovascular outcomes?
- How does retatrutide perform in people with common comorbidities like sleep apnea, fatty liver, or high blood pressure?
If Phase 3 confirms strong efficacy with manageable side effects, retatrutide could become a major option in obesity treatment.
Access today: what patients can do now
Retatrutide is not available in pharmacies. You can only access it through legitimate clinical trials. If you are interested:
- Visit ClinicalTrials.gov and search for “retatrutide.”
- Ask your clinician if you might qualify for a study and whether it makes sense for you.
- While waiting, discuss approved options like Wegovy or Zepbound.
Practical guidance for patients and clinicians
- Review your goals (for example, a 10–15% weight loss, improving sleep apnea, or lowering HbA1c).
- Share your medical history, especially any history of pancreatitis or thyroid cancer in the family.
- Plan for regular follow‑ups to review side effects, adjust doses, and check labs.
- Pair medicine with lifestyle changes for better results and maintenance.
Frequently asked questions (FAQs)
What were the headline Phase 2 results?
At 48 weeks, the 12 mg group achieved about 24.2% average weight loss. Lower doses also led to meaningful weight loss.
What is the most common side effect?
Nausea during dose increases. It usually improves over time, especially with slower titration.
Is retatrutide approved by the FDA?
No. It is investigational and only available in clinical trials.
When will Phase 3 results be available?
Public results are expected in 2025–2026, depending on the specific studies. If results are positive, regulatory review could follow.
Can I get retatrutide online? I see offers on social media.
Avoid unregulated sellers. These offers may be unsafe or illegal. Talk to your clinician about clinical trials or approved alternatives.
What if I cannot tolerate the side effects?
Tell your care team. Slower titration or supportive care may help. Another medicine may be a better fit for you.
Will I keep the weight off?
Some weight regain can happen after stopping. Long‑term plans may include ongoing therapy, lifestyle changes, or both.
Does retatrutide help with blood pressure, cholesterol, or fatty liver?
Many people improved these measures in the study. Your clinician may track these outcomes if you join a trial.
Plain‑language glossary
- GLP‑1 (glucagon‑like peptide‑1): A hormone that helps control appetite and blood sugar.
- GIP (glucose‑dependent insulinotropic polypeptide): A hormone that helps the body release insulin after meals.
- Glucagon: A hormone that raises blood sugar; in this context, targeting its receptor may help increase energy use.
- Randomized, double‑blind, placebo‑controlled: A study design that reduces bias so we can trust the results.
- Titration: Increasing the dose slowly to help manage side effects.
What to ask your clinician
- Am I a candidate for a retatrutide clinical trial?
- Should I consider Wegovy or Zepbound now while trials continue?
- How will we manage side effects, and what is the plan if they are strong?
- What labs will we follow (HbA1c, lipids, liver tests, etc.)?
References
- NEJM Phase 2 retatrutide obesity study (2023): https://www.nejm.org/doi/full/10.1056/NEJMoa2301972
- PubMed abstract: https://pubmed.ncbi.nlm.nih.gov/37366315/
- ClinicalTrials.gov Phase 3 (e.g., TRIUMPH program): https://clinicaltrials.gov/study/NCT05929066
- STEP 1 semaglutide obesity trial (NEJM 2021): https://www.nejm.org/doi/full/10.1056/NEJMoa2032183
- SURMOUNT‑1 tirzepatide obesity trial (NEJM 2022): https://www.nejm.org/doi/full/10.1056/NEJMoa2206038
- FDA drug information: https://www.fda.gov/drugs
Ready to explore medical weight management?
Consult with US-based telehealth providers to discuss FDA-approved GLP-1 medications and personalized obesity treatment plans.